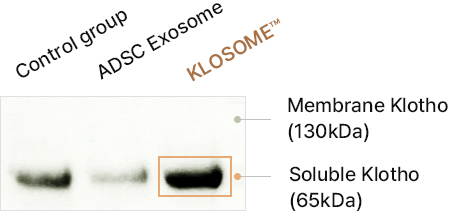
“Special stem cell exosome” encompasses not only the regenerative potential of stem cell exosomes for skin but also the anti-aging capabilities of Klotho, inhibiting the aging process of skin cells.
Klosome efficacy
Increase skin elasticity
Skin Regeneration
Wound healing
Sensitive and
inflamed skin
Pore reduction
Anti-aging

Klosome human safety
Klosome, EHLBIO’s next-generation skin booster has demonstrated proven safety in human trials.
Tested for dermatological
toxicity and irritation
Tested for skin cell regeneration
and elasticity efficacy
Tested safety in
human application
Tested for
melanocyte whitening efficacy
Listed on the International
Nomenclature Cosmetic Ingredient (INCI)
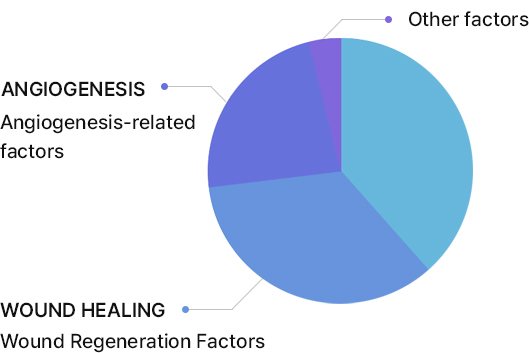
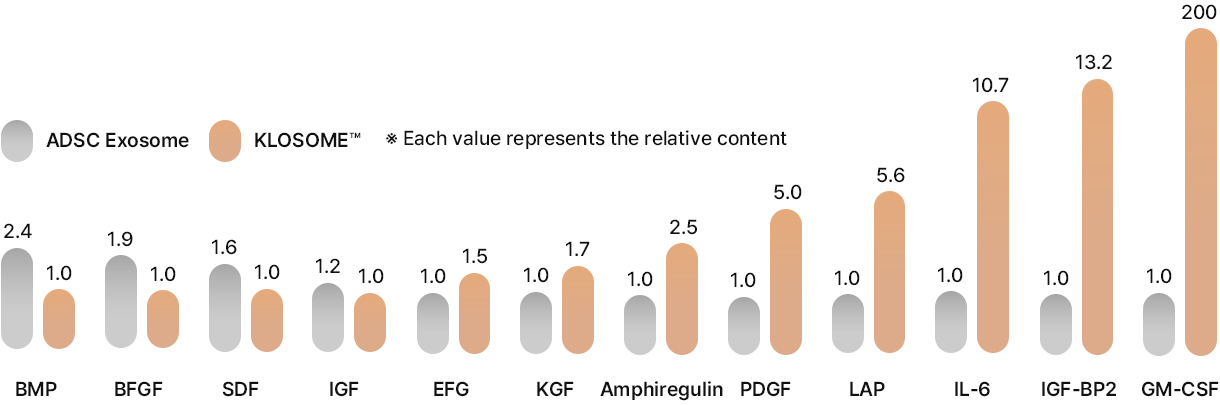
ADSC Exosome vs. KLOSOME ® Skin effective Factor Comparison Analysis
Main cytokines in Exosomes
Stem cell exosomes contain a large amount of immune regulatory factors that control inflammation and wound healing factors, facilitating tissue regeneration to aid in the recovery of damaged tissue.
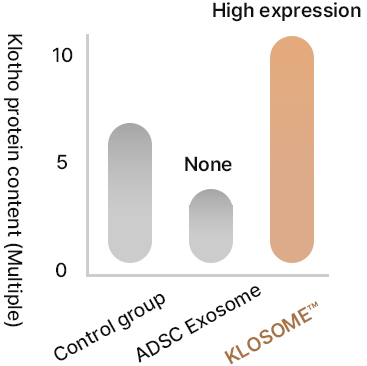
Comparison of protein content in Klosome
Comparison of main effective ingredient
As well as ADSC, KLOSOME contains various growth factors that promote the growth and differentiation of Keratinocyte and Fibroblasts in skin.
Especially, klosome® is found to contain abundant skin regeneration factors such as GM-CSF, IL-6, PDGF, and LAP, which contribute to tissue regeneration and scar prevention more effectively than ADSC exosome .
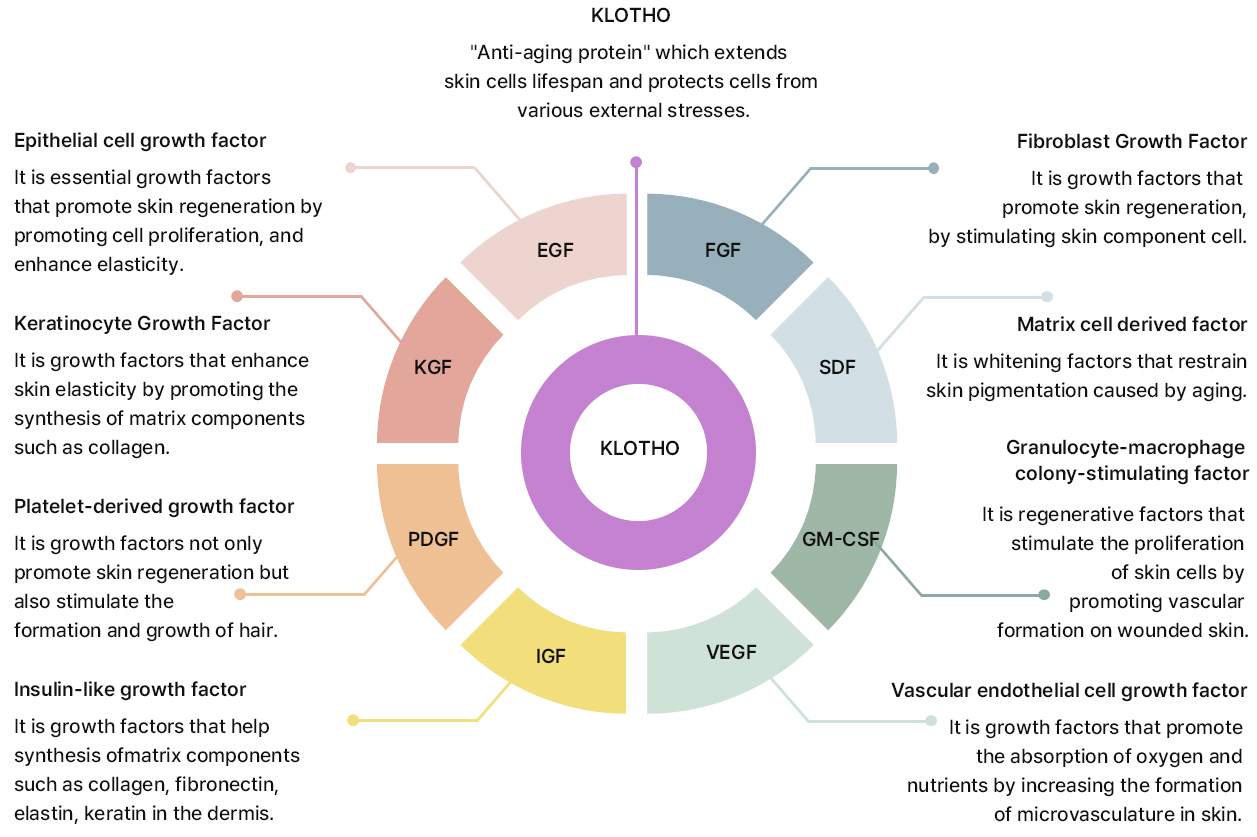
KLOSOME® Key Effective Ingredients
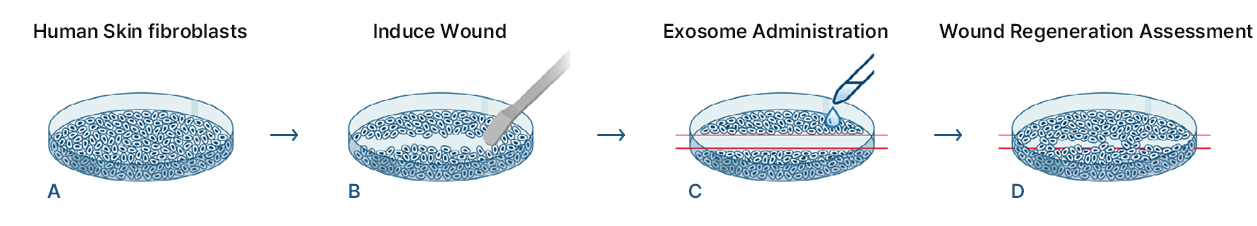
KLOSOME® Evaluation of efficacy in wound healing
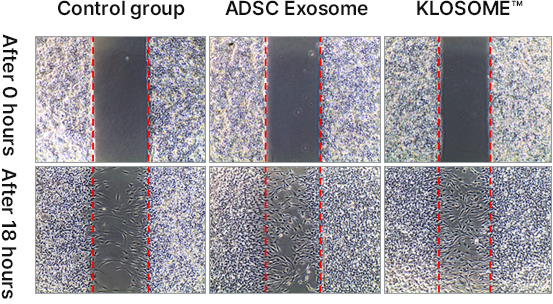
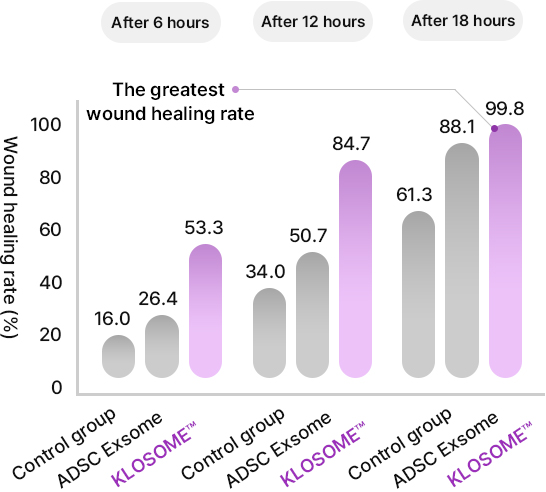
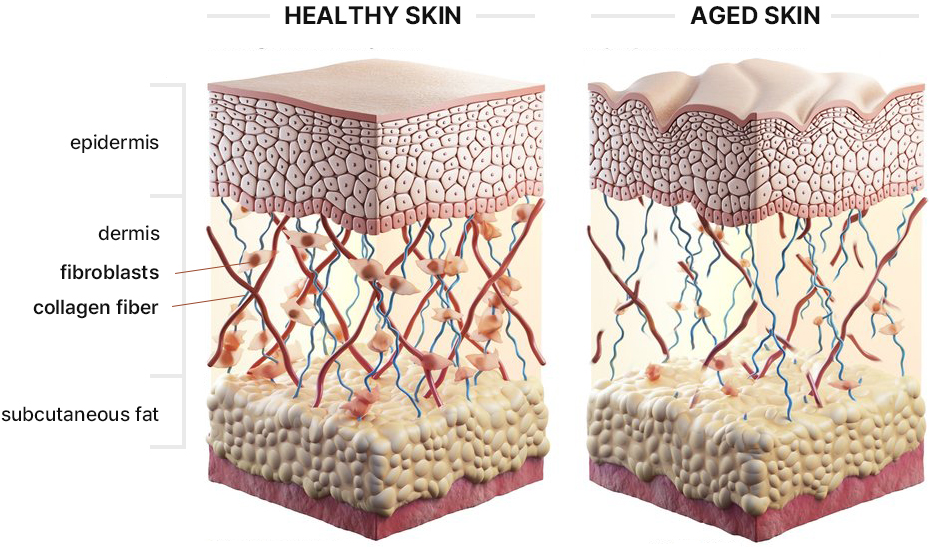
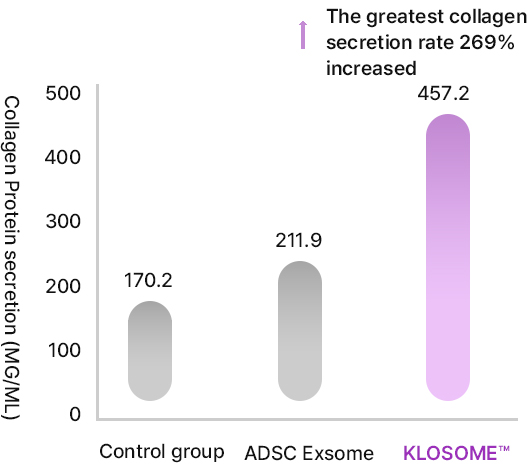
KLOSOME® Evaluation of efficacy in stimulating collagen secretion
- In healthy skin, fibroblasts in dermis secrete collagen to maintain elasticity
- When the skin ages, the number of fibroblasts decrease and form wrinkles due to the reduction in collagen.
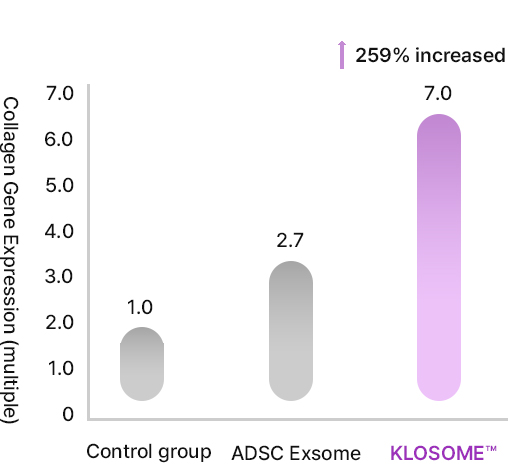
EHL BIO Klosome Patents and Papers
Klosome trademark registration
Proof of the efficacy of fibrosis inhibition published in International Academic Journals

Domestic Patent for Klotho Secretion Enhanced Stem Cells

Klosome Skin Regeneration (Wrinkle Improvement, Whitening Domestic Patent)
Phase 1 clinical trial approval from KFDA for chronic kidney disease patients

publication of anti-fibrotic efficacy in an international academic journal

Klosome trademark registration
Stem cell patent
Enhanced Klotho secretion stem cells
- Patent Application for September 29, 2021 (No. 10-2021-0129299)
- Patent Registration for April 6, 2022 (No. 1023854400000)
Exosome Patent
Cosmetic compositions for skin regeneration, wound healing, and whitening
- Patent Application for February 17, 2021 (No. 10-2021-0021026)
- Patent registration on December 15, 2021 (No. 1020210128902)
